Eye drops users have been issued a new warning from the Centers for Disease Control and Prevention (CDC) about a rare and dangerous bacterial infection which has been detected in two brands of over the counter eye drops.
68 patients have now been identified across 16 states, with 3 confirmed deaths linked to the outbreak.
The rare infection is resistant to most antibiotics making it difficult for medical professionals to treat and extremely dangerous to patients and their vision.
The rare bacterial infection, Pseudomonas aeruginosa, which is typically caused from contaminated soil, has been found in two brands of eyedrops, causing loss of vision, loss of the eyeball and the loss of life in three people.
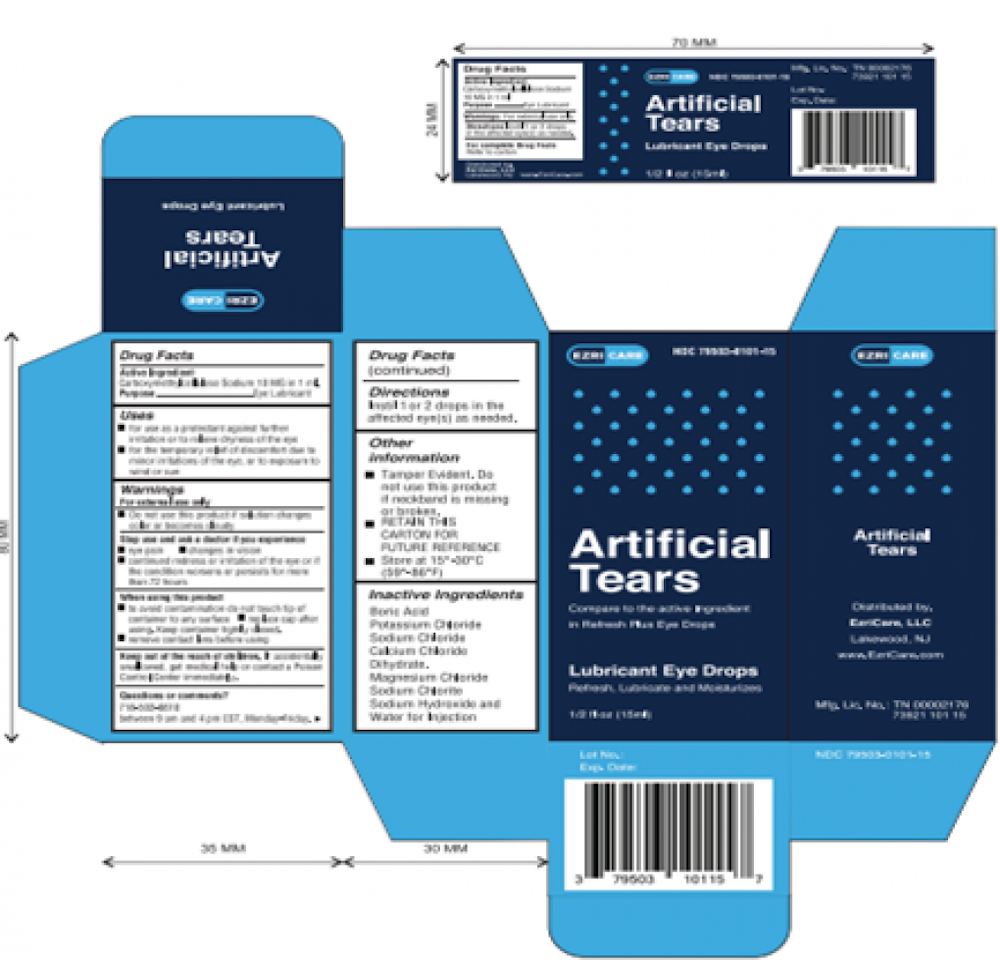
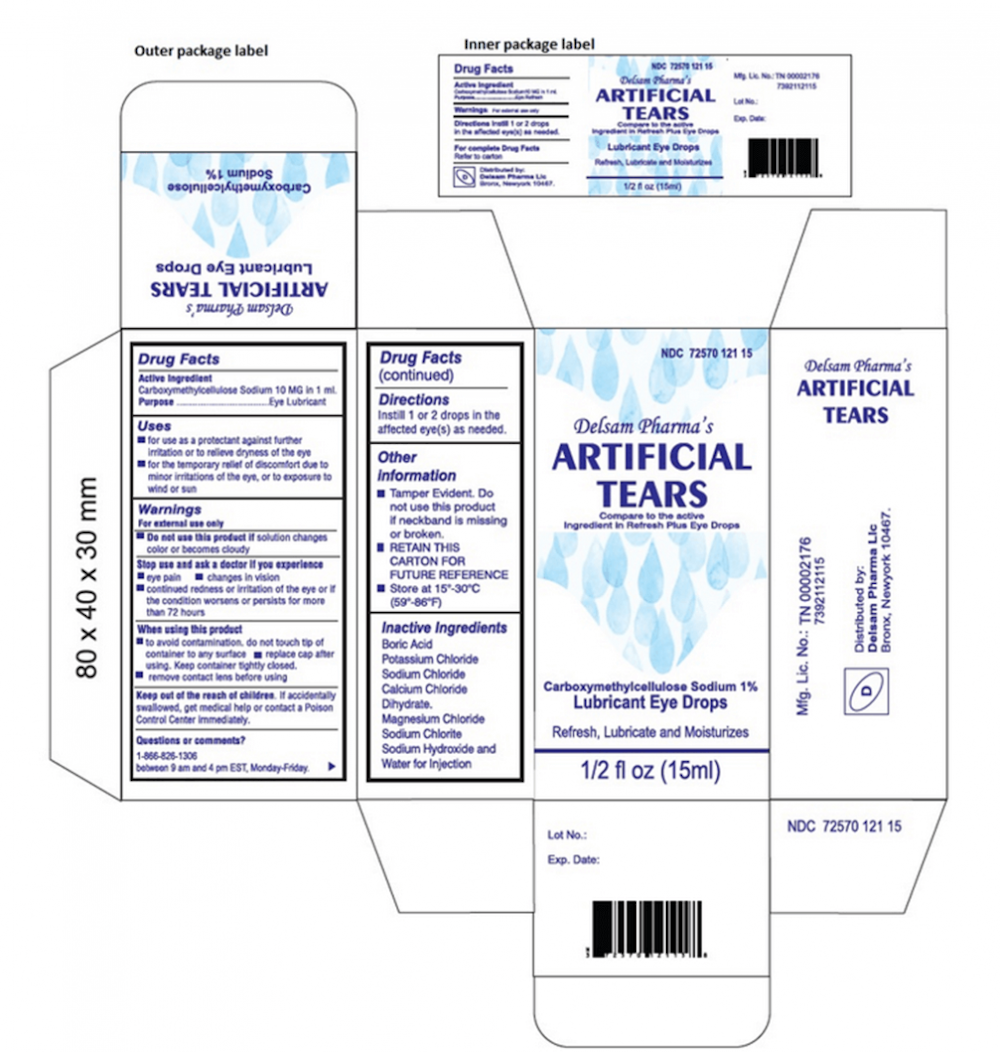
The two particular eye drop brands identified by the CDC and FDA are EzriCare Artificial Tears and Delsam Pharma's Artificial Tears.
Both brands were officially linked to the outbreak by the CDC in February. Both eye drop brands are owned by Global Pharma.
Ezricare have issued a Discontinue Use notice on their website for the Artificial Tears product and have issued a product withdrawal/recall with the FDA
Importance of CDC Warning & What to Do Next
- All use of the impacted brands (i.e. Artificial Tears brand from EzriCare or DelsamPharma) is stopped immediately. If you use any eye drops, you must check that the product is not listed currently by the FDA or CDC.
- If you are not a frequent user of eyedrops, it is important to search for any old bottles which might be in your house as these may be contaminated and will stay contaminated.
- If you have any of the symptoms listed below, you must immediately contact your doctor or healthcare professional
- Participate in the recall with the manufacturer (see below)
Symptoms
- Yellow, green or clear discharge from the eye
- Eye pain or discomfort
- Redness of the eye or eyelid
- Feeling of something in your eye (foreign body sensation)
- Increased sensitivity to light
- Blurry vision
CDC laboratory testing identified the presence of the outbreak strain in opened EzriCare bottles with different lot numbers collected from two states.CDC Official Health Advisory February 1, 2023
For customers, it is important to know that this product was sold across the USA and did not require a prescription. Therefore doctors / retailers won't be contacting customers as part of the recall. Consumers must do their own work to find the contaminated bottles.
The parent company, Global Pharma Healthcare, has said that it has informed its distributor network of the issue and of the official recall.
The company says that customers who have the recalled product in their possesssion should stop use of the product.

How To Identify 'Artificial Tears' Eyedrops
- Artificial Tears (carboxymethylcellulose sodium) Lubricant Eye Drops, 10 mg in 1 mL, ½ fl oz (15 ml)
Product Codes
- Ezricare NDC 79503-0101-15, UPC 3 79503 10115 7
- Delsam Pharma’s NDC 72570-121-15, UPC 3 72570 12115 8.
Recall Information
Consumers with questions regarding this recall can contact the manufacturers.
EzriCare
- Phone: Dial 1-516-715-5181 for Aru Pharma/Ezricare, LLC
- E-mail: Contact Aru Pharma/Ezricare, LLC at [email protected]
Delsma Pharma
- Phone: Dial 1-866-826-130
- E-mail: Contact Delsam Pharma LLC at [email protected]
Further information about the recall can be found at the FDA's website.
Packaging
The two products impacted by the recall are shown below.


Comments